-
What We Do
What we do
- Verticals
- Solutions
- Who We Are
As medical devices evolve into increasingly complex cyber-physical systems, test automation strategies are facing heightened regulatory scrutiny. Modern devices now combine embedded software, advanced electronics, and sophisticated visual user interfaces—yet many verification and validation practices still rely on legacy approaches never designed to assess such multi-dimensional behaviours. From the perspective of FDA Design Controls and international standards such as IEC 62304, IEC 60601, and ISO 13485, this growing disconnect introduces a significant and often underestimated compliance risk.
Under FDA 21 CFR Part 820 and ISO 13485, manufacturers are required to demonstrate that medical devices meet defined Essential Performance Parameters (EPPs) under intended operating conditions. This expectation extends beyond isolated electrical, mechanical or software outputs and into system-level behaviours, including visual indicators, user interfaces, and timing-dependent responses.
Similarly, IEC 60601 places strong emphasis on validating safety and essential performance at the system level, while IEC 62304 requires controlled, repeatable verification of software-driven behaviours. When visual confirmation of system states, alarms, or user interface behaviours remains manual or subjective, both traceability and repeatability are compromised. The result is test evidence that is difficult to reproduce, scale, or defend—creating avoidable friction during audits, inspections, and design reviews.
Conventional tests typically focus on measurable electrical signals or software logs and visual validation such as display content, LED states, motion sequences, or timing indicators which is often handled manually or semi-automatically.
Consider a reusable, electronic injection device that provides real-time, on-device visual feedback during dose delivery. The device may indicate injection progress through LEDs, on-screen graphics, or color-coded status indicators that change based on plunger position, delivery rate, occlusion detection, or user interaction. Under IEC 60601, the correct visual presentation of these indicators is part of the device’s safety and essential performance, while IEC 62304 requires that the software logic driving each visual state be verified in a controlled and repeatable manner. In practice, validation of these behaviours is often performed through manual observation, an engineer confirms that the device “looks correct” during a test run and records the result. This makes it difficult to demonstrate consistency across firmware versions, dose configurations or environmental conditions, weakening traceability and increasing risk during audits and inspections.
In regulated environments, these gaps can lead to nonconformities, audit observations, or delayed approvals.
Vision analytics based automated validation addresses this limitation by converting visual behaviours into quantifiable, machine-verifiable data. Using real-time video analytics, the system captures and analyses visual outputs such as display updates, LED behaviours, motion, and temporal sequencing. When integrated with data acquisition and control (DAC) and test equipment interfaces (Serial and Ethernet), visual validation can be synchronized with electrical measurements and system inputs. This enables true multi-dimensional correlation, aligning with regulatory expectations for system-level validation.
From a compliance perspective, this approach strengthens:
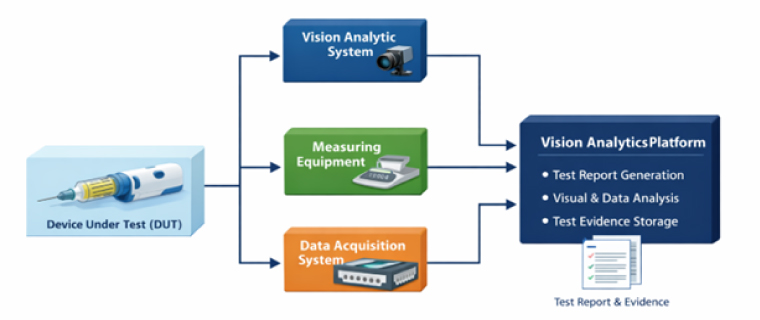
Figure 1 Vision Analytics Based Test System – Block Diagram
Vision analytics–driven test platform directly supports multiple regulatory frameworks:
AI-driven configuration and automated reporting further reduce human-dependent variability, one of the most common sources of compliance drift in long-term device maintenance.
As regulatory bodies increasingly evaluate how validation is performed and not just the results, manual and loosely integrated test approaches are becoming harder to defend. Vision analytics-based test approach provides a structured, objective, and repeatable validation methodology aligned with modern regulatory expectations.
It shifts test platform from a productivity tool to a compliance-enabling system. When the test is designed to observe and validate system behaviours at the same level of abstraction as the end user, it begins to generate evidence that is both technically meaningful and regulatory ready. Instead of simply asserting that a function was called or a signal changed, the automation verifies that the correct system state was reached, the expected visual feedback was presented, and the behaviours remained consistent across runs and configurations.
In today’s regulatory environment, test automation is inseparable from compliance strategy. Vision analytics–based test solutions offer a technically robust and regulation-aligned approach to validating modern medical devices - reducing risk, improving repeatability, and strengthening regulatory submissions.
For organizations preparing for future audits and increasingly complex devices, the evolution of test approach to vision analytics bases solution is no longer optional.